Research
The Three Pillars of Science
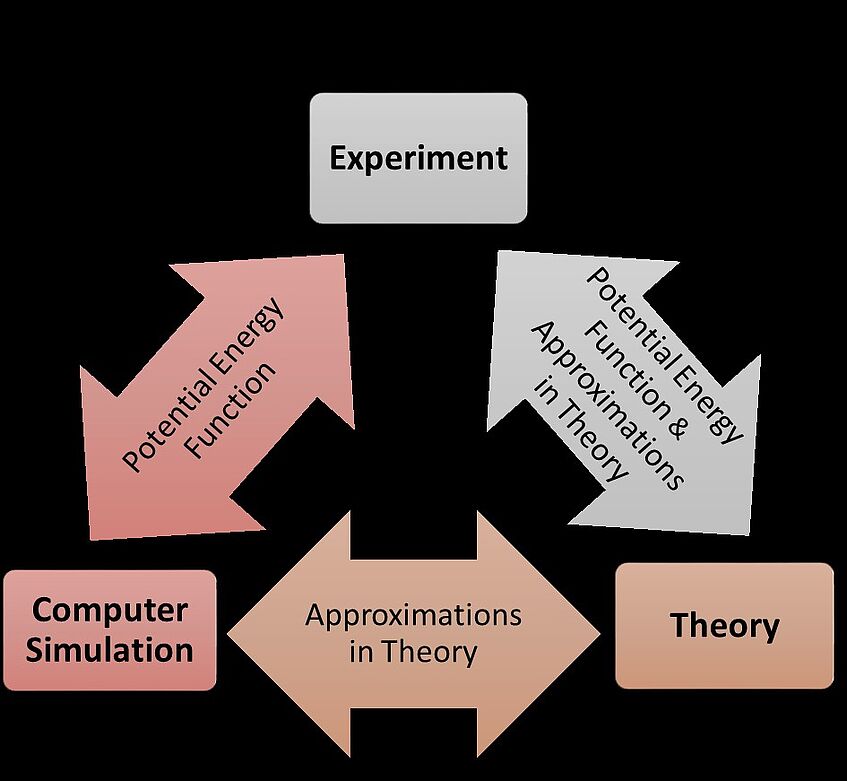
The Three Pillars of Science
The three pillars of science: experiment, theory and computer simulation. The double-headed arrows indicate possible, fundamentally important, comparisons which will contribute to a deeper understanding of the role of approximations concerning interaction energies and theoretical models: E. Wilhelm, J. Solution Chem. 43, 525-576 (2014).
Thermodynamics: A Few Selected Important Relations
Fundamental property relations or GIBBS equations:
d(nU)=Td(nS)−Pd(nV)+∑iμidni
↓ LEGENDRE transformation
d(nG)=−(nS)dT+(nV)dP+∑iμidni
General GIBBS-DUHEM equation:
M...molar thermodynamic quantity (homogeneous phase)
Mi≡[δ(nM)/δni]T,P,n_j≠i ... partial molar thermodynamic quantity
(δM/δT)P,{x_i}dT+(δM/δP)−∑ixidMi=0
LEWIS-RANDALL (LR) rule and HENRY’s law (HL): for liquid solutions, two components i and j, we have the measurable quantities:
fi(T,P,{xi}) ... fugacity of component i in solution
fi*(T,P) ... fugacity of pure component i
hi,j(T,P) ... HENRY fugacity (or HENRY's law constant) of i dissolved in j
Solubility of methane in water: temperature dependence of the Henry fugacity from the triple point of water to ca. 525 K
Plot of ln[h2,1(T,Ps,1)/GPa] against temperature T for methane dissolved in liquid water. h2,1(T,Ps,1) denotes the Henry fugacity (Henry's law constant) at temperature T and vapor pressure Pσ,1(T).
- expermental results of Rettich, Handa, Battino and Wilhelm, in J.Phys.Chem. 85, 3230-3237 (1981): the average percentage deviation of the Henry fugacity from the value calculated via the correlating BK function is about ±0.05%;
- experimental results of Crovetto et al. J.Chem.Phys. 76, 1077-1086 (1982): the average percentage deviation of the Henry fugacity from the value calculated via the correlating BK function is about ±2% after anchoring their data set onto our high-precision results.
The temperature where h2,1(T,Ps,1) vs. T shows a maximum is Tmax; 362K. That is, the partial molar enthalpy change on solution
ΔH2∞(T,Ps,1)≅–Tdln[h2,1(T,Ps,1)]/dT
of methane dissolved in water goes from negative values at low temperatures to positive values a elevated temperatures, and passes through zero at Tmax, in excellent agreement with direct calorimetric measurements (Wilhelm and Battino: Enthalpy Changes on Solution of Gases in Liquids, in Enthalpy and Internal Energy: Liquids, Solutions and Vapours, eds. E. Wilhelm and T. M. Letcher, The Royal Society of Chemistry/IACT, Cambridge, UK, 2018, ch. 10, pp. 269-298). Similar behavior is observed for the rare gases dissolved in water.
Summary
Various intellectually attractive topics concerning fluid nonelectrolyte solutions (liquids, vapors, gases) are investigated experimentally and theoretically, frequently leading to practical applications. Paraphrasing Mr. Spock, I find the field fascinating, and hope you agree!
⇒ cf. the list of publications (> 190 papers) ⇐
- Motivation: theoretical and practical roots (chemical engineering aspects).
- Thermodynamics: exact and/or asymptotically exact relations are used, together with theory-based approximations.
- Data reduction: general approaches, including the determination or estimation of important auxiliary quantities, such as partial molar volumes or second virial coefficients.
- Experimental results & scientific profit: For instance, high-precision Henry fugacities h2,1(T,Pσ,1), partial molar enthalpy changes ΔH2∞(T,Pσ,1) and heat capacity changes ΔCP,2∞(T,Pσ,1) on solution (infinite dilution) of gases in liquid water establish equivalency of
van’t Hoff analysis ⇔ calorimetry
- Models: based on Molecular Thermodynamics ⇒ frequently also of relevance for biophysical chemistry
⇓
Der einzige sichere Führer auf dem Weg der weiteren Entwicklung bleibt stets die Messung…
Max Planck, 1926
Acknowledgments: I thank all collaborators (from A, F, I, E, USA, Japan,…) for many years of agreeable collaboration, but three in particular: Profs. R. Battino (USA), J.-P. E. Grolier (F) and A. Asenbaum (A); and, of course, the funding agencies for providing monetary support: Fulbright Commission, FWF Austria, Austrian-French Scientific-Technical Cooperation Programs (for more than 25 years!), USA Public Health Service, NSF,…
The complexity of the liquid state and the large differences between our simple models and the actual solutions of molecules of a variety of sizes, shapes and force fields leaves ample room for further study.
The entire history of chemistry bears witness to the extraordinary importance of the phenomena of solubility.
Joel H. Hildebrand and Robert L. Scott
The Solubility of Nonelectrolytes
3rd edition, Reinhold, New York, 1950
⇓
Cutting-edge topical books supported by IUPAC and IACT (International Association of Chemical Thermodynamics, successor of IUPAC Commission I.2, Thermodynamics)
⇓
(1) HEAT CAPACITIES: Liquids, Solutions and Vapours (2010)
(2) VOLUME PROPERTIES: Liquids, Solutions and Vapours (2015)
(3) ENTHALPY AND INTERNAL ENERGY: Liquids, Solutions and Vapours (2018)
Editors: Emmerich Wilhelm; University of Wien, Institute of Materials Chemistry & Research/ Institute of
Physical Chemistry, Wien (Vienna), Austria
Trevor M. Letcher; University of KwaZulu-Natal, Durban, South Africa
Publisher: The Royal Society of Chemistry, Cambridge, UK.
